Equipment Wipes and Sprays
The Azowipe® Equipment range has been designed with non-invasive medical devices in mind. 70% Isopropanol Alcohol provides convenient, effective, and rapid disinfection of equipment. Azo™ Equipment 70% IPA combined with an effective non-woven substrate creates a powerful disinfectant with fast kill rates, offering high bactericidal, virucidal, fungicidal and yeasticidal efficacy.
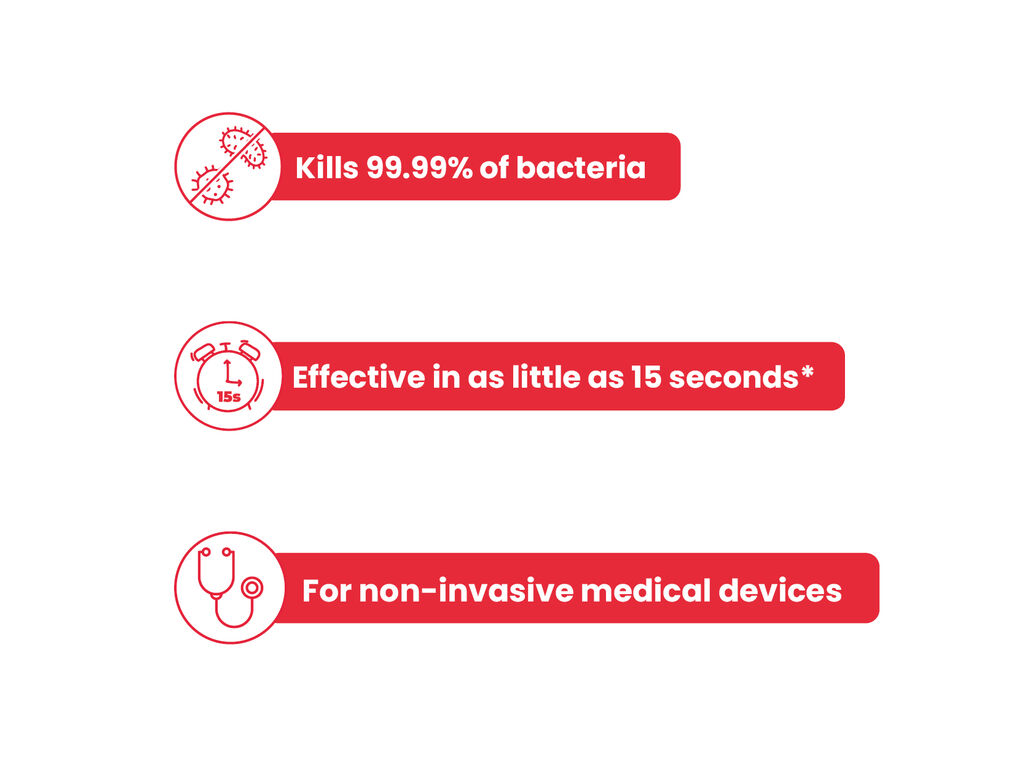
Our Azowipe® Equipment range is clinically proven to kill 99.99% of bacteria including MRSA, Psuedomonas aeurginosa, Candida albicans, HIV, Hep B, Hep C and E. coli from 15 seconds. The wipe is made from 70% v/v IPA blended with 0.2 micron deionised water, leaving no residue behind and allowing the disinfected area of equipment to be used shortly after application.
The Azowipe® Equipment is also compliant to directive 93/42/EEC and are manufactured to ISO 13485 standards.
Our Azowipe® Equipment range is clinically proven to kill 99.99% of bacteria including MRSA, Psuedomonas aeurginosa, Candida albicans, HIV, Hep B, Hep C and E. coli from 15 seconds. The wipe is made from 70% v/v IPA blended with 0.2 micron deionised water, leaving no residue behind and allowing the disinfected area of equipment to be used shortly after application.
The Azowipe® Equipment is also compliant to directive 93/42/EEC and are manufactured to ISO 13485 standards.
- Designed for non-invasive medical devices
- Kills 99.9% of bacteria
Clinically tested to kill most bacteria, helping to prevent infections. - Effective within 15 seconds
Designed to kill certain bacterias with a contact time of just 15 seconds - Class IIa medical device compliant
Azowipe® Equipment range is Class IIa medical device compliant to directive 93/42/EEC and is manufactured to ISO 13485 and ISO 9001 standards. - Quick drying no residue formulation
Offering a quick and easy cleaning solution before and after each patient. - Manufactured in the UK
Our full range is manufactured in the UK, offering high standards and supply chain resilience.
Product Enquiry
Product Name: Azo™ 70% Ipa Disinfectant Wipes CE 200 Wipes
Product Code: 81138
Product Details
Azo™ Alcohol Equipment Disinfectant Wipes CE – 200 Wipes
Product
Code
81138
Type
Canister
Pack
Quantity
200
Case
Breakdown
12 canisters per case

Azo™ Alcohol Equipment Wipes CE – 100 Wipes
Product
Code
AZ100WNCE
Type
Canister
Pack
Quantity
–
Case
Breakdown
12 canisters per case

Azowipe® Alcohol Equipment Wipes CE – 250 Wipes
Product
Code
AZ250WCE
Type
–
Pack
Quantity
–
Case
Breakdown
1 X 12 canisters per case

Product Efficacy
| European Standard | Microorganism | Contact Time |
|---|---|---|
| ASTM E1052 | Duck Hepatitis Virus (HEP B surrogate) | 15s |
| EN 13624:2013 | Candida albicans | 15s |
| EN 13697:2015 | Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureu, Enterococcus hira | 60s |
| EN 13727:2012 | Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus hirae, Acinetobacter baumannii, Klebsiella pneumonia, Vancomycin Resistant Enterococcus (VRE), MRSA | 15s |
| EN 14348:2005 | Mycobacterium terrae | 60s |
| EN 14476:2013 | Murine norovirus | 60s |
| EN 14476:2013 | Bovine viral diarrhoea virus (Hep C surrogate), Vaccinia virus (HIV), Feline coronavirus (MERS Coronavirus), Influenza A H1N1 | 15s |
| EN 14561:2006 | Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus hirae | 60s |
| EN 14562:2006 | Candida albicans, Aspergillus brasiliensis | 60s |
| EN 16615:2015 | Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus hirae, Candida albicans | 60s |
| EN 1650 2008 | Candida albicans ATCC 10231 | 15s |
| EN 1276 200 | Legionella pneumophila, Campylobacter jejuni, Listeria monocytogenes, Salmonella enterica, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Enterococcus hirae | 15s |
Explore the Infection Prevention range

Universal Wipes and Sprays